Surface Energy
Surface Energy: Overview
This topic covers concepts, such as, Intermolecular Forces,Molecular Range of Intermolecular Forces,Sphere of Influence etc.
Important Questions on Surface Energy
How much work is required to form a bubble of radius from the soap solution having surface tension ?
Derive the dimensional formula of surface energy.
A certain number of spherical drops of a liquid of radius coalesce to form a single drop of radius and volume If is the surface tension of the liquid then:
If the excess pressure inside a soap bubble is balanced by oil column of height , then the surface tension of soap solution will be
What should be the pressure inside a small air bubble of radius situated just below the surface of water? Surface tension of water N/m and atmospheric pressure .
The surface tension of soap solution is . The work done in blowing to from a soap bubble of surface area
On the surface of the liquid in equilibrium, molecules of the liquid possess
When a number of small droplets combine to form a large drop, then
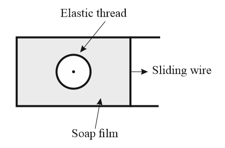
The figure shows a soap film in which a closed elastic thread is lying. The film inside the thread is pricked. Now, the sliding wire is moved out so that the surface area increases. The radius of the circle formed by elastic thread will,
Two droplets coalesce in a single drop. In this process _____.
The total energy of the free surface of a liquid drop is times the surface tension of the liquid. What is the diameter of the drop? (Assume all terms in SI units)
Eight droplets of water, each of radius , coalesce into a single drop. Find the change in total surface energy. (Surface tension of water
A mercury drop of radius falls from a height on a glass plate and breaks up into a million droplets, all of the same size. Find the height from which the drop must have fallen. (Density of mercury, Surface tension of mercury
There is a soap film on a rectangular frame of wire of area . If we increase the area of the frame to , find the work done in the process. (Surface tension of soap film
Why do molecules of a liquid lying in the surface film posses extra energy?
Explain surface tension on the basis of the molecular theory.
Define sphere of influence.
Define range of molecular attraction.
Cotyledons are also called-
